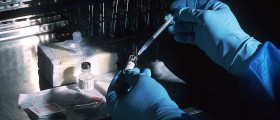
US pharmaceutical giant Merck has announced that they are stopping clinical test of the HIV vaccine because it has not given positive results. There were many high hopes over the V520 vaccine ever since it was introduced especially because it showed promise all the way through but failed in a big trial to prevent infection or reduce the amount of the virus in the bloodstream.
While other traditional vaccines were made to cause the immune system to produce antibodies, the V520 was made to stimulate T cells, a component of the immune system.
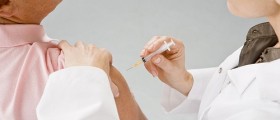
The trial was launched in 2004 and included 3,000 volunteers from the United States and Latin American countries from between 18 and 45 years of age who were at high risk of infection of HIV.
Smaller experiments done on animals and humans had provided encouraging results and prompting Merck and US researchers to develop the vaccine that would use a common cold virus to deliver three genes from the AIDS virus. However, this new strategy didn’t prove effective in the larger clinical trial and finding an effective AIDS vaccine will remain one of the most challenging tasks facing researchers and modern medicine.
Merck and the US researchers will share details of the trial with other scientists and in that way provide critical insights into this disease and future vaccine development.
While other traditional vaccines were made to cause the immune system to produce antibodies, the V520 was made to stimulate T cells, a component of the immune system.
The trial was launched in 2004 and included 3,000 volunteers from the United States and Latin American countries from between 18 and 45 years of age who were at high risk of infection of HIV.
Smaller experiments done on animals and humans had provided encouraging results and prompting Merck and US researchers to develop the vaccine that would use a common cold virus to deliver three genes from the AIDS virus. However, this new strategy didn’t prove effective in the larger clinical trial and finding an effective AIDS vaccine will remain one of the most challenging tasks facing researchers and modern medicine.
Merck and the US researchers will share details of the trial with other scientists and in that way provide critical insights into this disease and future vaccine development.
Loading...



_f_280x120.jpg)