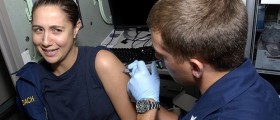
A vaccine that blocks infection by the four virus types that cause most cervical cancers and genital warts appears safe and effective, but may actually increase the chance of disease in some patients, according to Food and Drug Administration documents.
An FDA panel of outside experts is to meet Thursday and discuss whether to recommend that the agency approve the vaccine. Merck said the vaccine has the potential to reduce the annual number of new cervical cancers worldwide to roughly 150,000, from the current 500,000, and cut global deaths from the cancer by more than two-thirds, to an estimated 90,000.
The first is that the vaccine may lead to an increased number of cases of a cancer precursor among patients already infected by any of the four virus types at the time they receive the vaccine, and whose immune systems have not cleared the virus from their bodies.
The second concern is that any advantage the vaccine provides in protecting against the four virus types could be offset by infection by any of the multiple other types of HPV that the vaccine does not cover, according to the FDA documents.
An FDA decision is expected by June 8. Should it approve it, the national Advisory Committee on Immunization Practices will decide later that month whether to endorse routine vaccination with the vaccine. The committee's HPV vaccine workgroup is recommending the vaccine be given to girls 11 and 12, and the committee will consider recommendations for females 13 to 26.I
An FDA panel of outside experts is to meet Thursday and discuss whether to recommend that the agency approve the vaccine. Merck said the vaccine has the potential to reduce the annual number of new cervical cancers worldwide to roughly 150,000, from the current 500,000, and cut global deaths from the cancer by more than two-thirds, to an estimated 90,000.
The first is that the vaccine may lead to an increased number of cases of a cancer precursor among patients already infected by any of the four virus types at the time they receive the vaccine, and whose immune systems have not cleared the virus from their bodies.
The second concern is that any advantage the vaccine provides in protecting against the four virus types could be offset by infection by any of the multiple other types of HPV that the vaccine does not cover, according to the FDA documents.
An FDA decision is expected by June 8. Should it approve it, the national Advisory Committee on Immunization Practices will decide later that month whether to endorse routine vaccination with the vaccine. The committee's HPV vaccine workgroup is recommending the vaccine be given to girls 11 and 12, and the committee will consider recommendations for females 13 to 26.I
Loading...




_f_280x120.jpg)