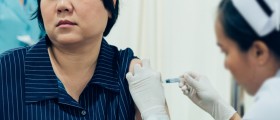
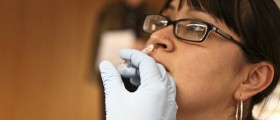
It’s reported that experimental cervical cancer vaccine remains effective for nearly five years and may protect women against more types of cancer-causing viruses than was originally thought.
On the online issue of The Lancet it is today reported that more than 1,100 women participated in a study gauging the effectiveness of the vaccine called Cervarix, under development by drug GlaxoSmithKline. 776 of those women were tracked and followed for 53 months and in this period they received either three doses of the vaccine or three doses of placebo.
A specific type of infectious pathogen, called the human papilloma virus (HPV), is credited with causing most cervical cancers. Cervarix is designed to protect against HPV types 16 and 18, which account for about 70 percent of cervical malignancies.
The team of researchers found another vaccine which can confer protection against two other HPV types, 45 and 31, that can also trigger the cancer.
After 53-month-examination, all the women have been tested positive for being immunized with HPV 16 and 18, the level of antibodies has not been decreased and the protection against the cancer-causing viruses continued.
Women who got Cervarix did not report any further problems comparing to the ones that got placebo.
Rachel Winer, University of Washington, Seattle, research scientist and HPV researcher, said that the vaccine’s long-term effectiveness is promising and once it hits the market, young women in their late adolescence or early 20s would be the most likely candidates to receive it.
Besides, there is another vaccine for cervical cancer that is waiting approval. It is called Gardasil and it protects against HPV 16 and 18, and also against HPV 6 and 11, which Skidmore said accounts for about 90 percent of genital warts.
On the online issue of The Lancet it is today reported that more than 1,100 women participated in a study gauging the effectiveness of the vaccine called Cervarix, under development by drug GlaxoSmithKline. 776 of those women were tracked and followed for 53 months and in this period they received either three doses of the vaccine or three doses of placebo.
A specific type of infectious pathogen, called the human papilloma virus (HPV), is credited with causing most cervical cancers. Cervarix is designed to protect against HPV types 16 and 18, which account for about 70 percent of cervical malignancies.
The team of researchers found another vaccine which can confer protection against two other HPV types, 45 and 31, that can also trigger the cancer.
After 53-month-examination, all the women have been tested positive for being immunized with HPV 16 and 18, the level of antibodies has not been decreased and the protection against the cancer-causing viruses continued.
Women who got Cervarix did not report any further problems comparing to the ones that got placebo.
Rachel Winer, University of Washington, Seattle, research scientist and HPV researcher, said that the vaccine’s long-term effectiveness is promising and once it hits the market, young women in their late adolescence or early 20s would be the most likely candidates to receive it.
Besides, there is another vaccine for cervical cancer that is waiting approval. It is called Gardasil and it protects against HPV 16 and 18, and also against HPV 6 and 11, which Skidmore said accounts for about 90 percent of genital warts.
Loading...


_f_280x120.jpg)