Arguably, HIV is a largest pandemic in human history. Since HIV was confirmed as the cause of AIDS in 1984, more than 35 million people have died and the virus continues to steadily spread worldwide. In Sub-Saharan Africa, the most affected area in the world, 1 in every 20 adults is infected.
Modern antiretroviral drugs transformed HIV treatment and management
In spite of the severity and propagation of this infection, to this day there is no cure for HIV. However, the advent of combination antiretroviral therapy (ART) has improved quality of life and life expectancy for patients infected with HIV.
Combined, they target almost every step of the viral life cycle. This has revolutionized the lives of affected patients globally, making HIV infection a chronic, manageable disease, instead of the fatal infection it once was.
HIV pandemic continues to grow
Although the knowledge about the routes of HIV transmission is greater than ever, putting an end to the HIV pandemic seems hardly achievable, with more than 7,000 new cases of infection being registered every day. Furthermore, ART therapy can take a huge financial toll on both patients and healthcare systems. This is aggravated by the fact that HIV infection is more common in the developing countries where access to treatment is frequently impaired and economic sustainability is a full-time problem.
ART cannot fully eradicate virus
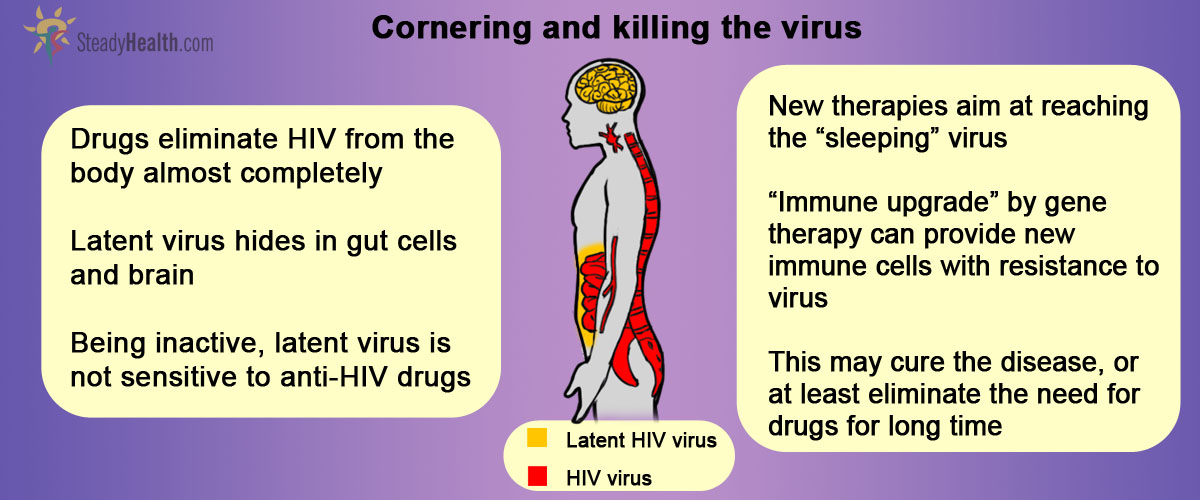
Why it is that ART therapy cannot eradicate the HIV infection completely, though? At the root of the answer for this question lies what is called HIV latency. HIV latency refers to the rare but extremely stable viral reservoir formed within the so-called resting memory CD4+ T cells.
CD4+ T cells are the cells of immune system where the HIV virus normally resides. Newly produced (“naïve”) CD4+ T cells get exposed to various antigens (harmful pathogens coming from our environment) and become activated. This activation triggers a chain of events that result in the elimination of pathogen. For the most part, the activated T cells die within weeks of the encounter with the antigen. A minority, though, reverts to a resting state and persist as memory T cells which give the immune system the capability of responding more immediately to the same antigen in the future. It is precisely within these cells that latent HIV viruses form reservoirs. Because these cells persist in the body for long periods of time, the latent virus can be maintained indefinitely, away from the action of regular antiretroviral drugs.
See Also: Breaking News! Potential HIV Cure Found - A Protein that Entombs HIV
This constitutes, then, a major obstacle to achieving cure.
Can The Problem Of Latent HIV Virus Be Addressed?
At the anatomical level, the tissue belonging to the lymphatic system, namely that which is associated with the organs from the gut, is home for the major HIV reservoir, while the brain is the most worrisome reservoir, as the drugs diffusion to the brain is limited.
Multiple molecular mechanisms are at play in HIV survival, and devising a therapeutic strategy that effectively eliminates both replicating and latent viral load is undoubtedly a challenging endeavor. But hopes are up, as the possibility of resorting to gene therapy to boost the immune system’s capability of resisting to the virus does not seem unreasonable or out of place.
“Berlin patient”
The first step for this sort of therapy was given in 2007, when the so-called “Berlin patient” – HIV positive and diagnosed with acute myeloid leukemia – received a bone marrow transplant from a donor that turned out to possess CCR5 ∆32 mutation. This means that this donor’s DNA sequence had a very rare mutation in the gene responsible for the protein called CCR5. What is the relevance of this? The CCR5 protein is present on the surface of T cells and HIV enters these cells by interacting with the CCR5 protein. The genetic framework that the donor had leads to the reduced expression of the CCR5 surface proteins. Without those, the virus cannot enter the patients’ cells, thus rendering them resistant. The “Berlin patient” discontinued ART the day before the first transplantation and, after 6 years of follow up in the absence of therapy, he shows no trace of HIV in blood and tissue samples, revealing no evidence for persistent HIV. Levels of HIV-specific antibodies have also declined, suggesting that HIV antigen stimulation was very low or absent after transplantation.
Furthermore, the suitable donors are rare, what makes the search for a compatible donor with the protective genotype an additional challenge. Nevertheless, the success of the “Berlin patient” spiked the scientific community’s interest in generating HIV-resistant cells or deleting the virus from infected cells through gene therapy.
Gene therapy has a great potential for eliminating HIV
Several approaches for gene therapy are now studied, and one of the most promising is exactly the one used by the scientists from the University of Pennsylvania. These investigators removed the patients’ CD4+ T cells and manipulate them, so that they expressed a protein called engineered zinc-finger nuclease (ZFN). The premise here, supported by previous studies in mouse models, was that ZFN could inactive the CCR5 protein in normal human T cells, thus mimicking the CCR5 ∆32 mutation. In a way, ZFN has the potential to provide a pool of cells permanently resistant to HIV-1 infection. These patients’ modified immune cells were then infused back and they were monitored for their infection and other safety-related parameters.
See Also: GMO Cells: A Revolutionary HIV Treatment
Of the 12 patients studied in the University of Pennsylvania Phase I trial, 6 were off antiretroviral therapy and 6 remained on treatment. Infusions were safe and tolerable and viral loads dropped in four patients whose treatment was interrupted for 12 weeks. For one of those, the viral loads were below the limit of detection. It was later discovered that this patient was heterozygous for the CCR5 ∆32 mutation, i.e., half of his CCR5 genes are naturally disrupted, which provided some interesting insight for researchers. Also, modified T cells were still detected in the blood weeks after infusions had been terminated, and they were even observed in the gut-associated lymphoid tissue (mentioned above as one of the main reservoirs for latent viruses), suggesting that these cells travel normally in the body.
Although an important first step, larger studies are definitely needed. Let’s not forget that numerous mechanisms contribute to HIV persistence and, quite possibly, multiple approaches will be necessary to address the many aspects of HIV infection and the latent HIV reservoirs.
- DOUEK, D. C. 2013. Immune Activation, HIV Persistence, and the Cure. Topics in Antiviral Medicine, 21, 128-132
- PASSAESA, C. P. & SÁEZ-CIRIÓN, A. 2014. HIV cure research: Advances and prospects. Virology
- KENT, S. J., REECE, J. C., PETRAVIC, J., MARTYUSHEV, A., KRAMSKI, M., ROSE, R. D., COOPER, D. A., KELLEHER, A. D., EMERY, S., CAMERON, P. U., LEWIN, S. R. & DAVENPORT, M. P. 2013. The search for an HIV cure: tackling latent infection. Lancet Infectious Diseases, 13, 614-621
- LAFEUILLADE, A. & STEVENSON, M. 2011. The Search for a Cure for Persistent HIV Infection. AIDS Reviews, 13, 63-66
- RUELAS, D. S. & GREENE, W. C. 2013. An Integrated Overview of HIV-1 Latency Cell, 155, 519-529.
- Mindmap by steadyhealth.com
- Photo courtesy of Lwp Kommunikáció by Flickr : www.flickr.com/photos/lwpkommunikacio/12971165295


Your thoughts on this