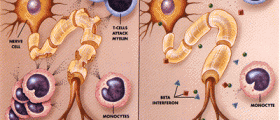
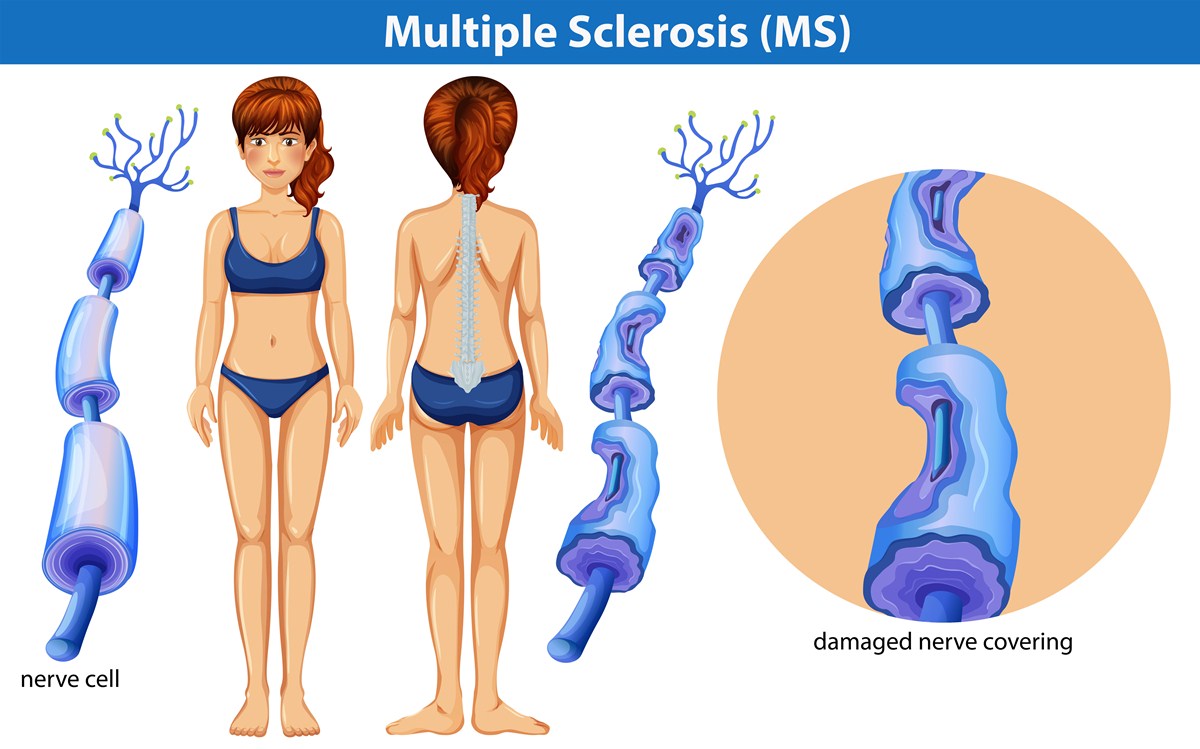
Multiple sclerosis is a chronic condition that can be very challenging for patients to deal with. This is a disorder of the central nervous system marked by weakness, speech impediments, visual disturbances and inability to control your bladder and typically affects women at twice the rate of men. Unfortunately, this is an autoimmune disease and worsens as the body continues to destroy the myelin fibers that help your nerves send communication signals between each other. [1]
One treatment that is thought to have an effect on this condition goes by the name of Tysabri (Natalizumab). This is considered to be a new generation of treatment options for multiple sclerosis and has a very high efficacy rate. Unfortunately, not all is good with this medication and there is a very complex girth of side effects that can make this therapy less than ideal.
This medication works by blocking a4 integrins. This is a compound that normally is very useful in the body because it helps cells attach together but becomes one of our biggest adversaries when we are diagnosed with multiple sclerosis. Because of the autoimmune nature of this condition, the body begins to attack to these molecules and the body degenerates as a result.
In one long-term study looking at how Tysabri works over a period of 5 years, patients with multiple sclerosis, the results were quite impressive. It was found that the rate of relapse of multiple sclerosis episodes decreased between 73 and 94 percent when patients took this medication. This is a very high level of effectiveness and results are better than those seen in older generation medications. [2]
Although results are promising, the issue that you need to consider is the risk of side effects that are seen with this medication. In multiple studies, it was determined that patients that took Tysabri were more likely to develop a type of cancer called progressive multifocal leukoencephalopathy (PML). Because of this effect, the manufacturers of this medication suspended the marketing of this product in order to have a more thorough review of the risk panel. It was reinserted into the market in 2006 after it was determined that the risks for PML were low enough to not outweigh the therapeutic benefits of this procedure. PML is a manifestation of a virus known as the John Cunningham virus (JC virus). This is a disease that affects 80 percent of the population but is clinically silent in the majority of cases.
Besides the possible risk of cancer, Tysabri is a well-tolerated medication in the short-term and patients will typically only have signs of pharyngitis, fatigue, anxiety and sinus congestion. It may be hepatotoxic so patients taking this medication also need to have liver function tests as part of routine blood checks to make sure the liver does not get too damaged.
Long-term complications include hypersensitivity reactions (allergic reactions), the potential of recurrent infections or other types of cancers are long-term risks that may cause the medication to be discontinued in patients. If you do stop this medication, studies show that patients typically will begin to have signs of multiple sclerosis resurfacing within 7 months of cessation of therapy. This is a reason why taking this medication is such a challenging decision for each patient. [3]
- 1.) https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0024315/
- 2.) https://www.ncbi.nlm.nih.gov/pubmed/27475049
- 3.) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3983075/
- Photo courtesy of SteadyHealth






-and-Multiple-Sclerosis-Differences-And-Similarities_f_280x120.jpg)