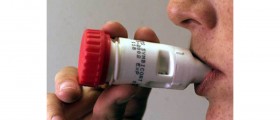
Merck & Co.'s top-selling asthma drug Singulair, also used to treat allergies, may be linked to mood and behavior changes as well as suicide. FDA started working on this matter with Whitehouse Station, N.J.-based Merck in order to go through the studies and patient reports. However, any final reports won’t be ready until nine months as this is how long the examination will take.
The company did not reveal how many deaths had occurred from Singulair usage. Singulair is the most prescribed respiratory drug in the US and has been on the market for 10 years. Last year, the company had revised the drug's prescribing information and included tremors, depression, suicide, and anxiousness as possible side effects.
The FDA advised doctors and patients to continue using Singulair until more information is available.
Head of Merck's Singulair development program, George Philip, reported in a phone interview that clinical studies showed no suicide risk. Suicide has only been reported in postmarketing experience in anecdotal reports. He also said that the number of suicides is "very small" relative to the amount of people taking the drug. How comforting is that?
Nevertheless, Merck plans to highlight these behavioural changes by talking with doctors and providing information leaflets for patients.
AstraZeneca PLC's Accolate and Critical Therapeutics Inc.'s Zyflo and Zyflo CR, three asthma medicines similar to Singulair will also be reviewed for suicide and behavioral changes.
In the last couple of years, regulators are closely watching for any drug-induced behavior changes and suicide risks. The list of suspected drugs is growing including antidepressants, and treatments for attention deficit hyperactivity disorder and epilepsy.
Last month, Pfizer Inc.'s antismoking drug Chantix has been linked to inducing psychiatric disorders after 39 patients committed suicide while taking the medicine.
The company did not reveal how many deaths had occurred from Singulair usage. Singulair is the most prescribed respiratory drug in the US and has been on the market for 10 years. Last year, the company had revised the drug's prescribing information and included tremors, depression, suicide, and anxiousness as possible side effects.
The FDA advised doctors and patients to continue using Singulair until more information is available.
Head of Merck's Singulair development program, George Philip, reported in a phone interview that clinical studies showed no suicide risk. Suicide has only been reported in postmarketing experience in anecdotal reports. He also said that the number of suicides is "very small" relative to the amount of people taking the drug. How comforting is that?
Nevertheless, Merck plans to highlight these behavioural changes by talking with doctors and providing information leaflets for patients.
AstraZeneca PLC's Accolate and Critical Therapeutics Inc.'s Zyflo and Zyflo CR, three asthma medicines similar to Singulair will also be reviewed for suicide and behavioral changes.
In the last couple of years, regulators are closely watching for any drug-induced behavior changes and suicide risks. The list of suspected drugs is growing including antidepressants, and treatments for attention deficit hyperactivity disorder and epilepsy.
Last month, Pfizer Inc.'s antismoking drug Chantix has been linked to inducing psychiatric disorders after 39 patients committed suicide while taking the medicine.
Loading...