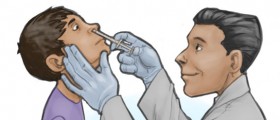
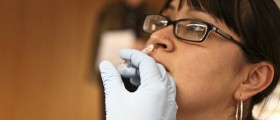
Spraying flu vaccine into the noses of babies and preschoolers offers significantly more protection than shots, says one of the largest comparisons of flu inoculations ever performed.
The study found the spray vaccine was 55 percent more effective than traditional flu shots when given to nearly 8,000 children under age 5.
The nasal spray FluMist, the only flu vaccine made of live but weakened influenza virus, now is sold only for children 5 and older. Manufacturer MedImmune Inc., which funded the new research, plans to seek government approval to sell FluMist for younger children as well.
And children are the prime spreaders of flu virus, fueling infections in older people who may not survive. Each winter, flu kills 36,000 Americans, most of them elderly.
“Our current thinking is that to control influenza, we really have to vaccinate all children,” said Dr. Robert Belshe, a prominent vaccine specialist at St. Louis University who led the new study. “Anything that makes it easier and more effective (to vaccinate) children is going to contribute a lot to the protection against influenza.”
The study did find a safety concern: A few of the very youngest patients, those ages 6 months to 2 years, had an episode of asthma-like wheezing in the weeks after the first FluMist dose.
The increased risk was slight — 1 percent more children wheezed after FluMist than after flu shots — and the reaction was temporary. But Belshe still is analyzing whether the risk would offset the increased flu protection, and regulators undoubtedly will ask whether it means FluMist should be used only after age 2.
The study found the spray vaccine was 55 percent more effective than traditional flu shots when given to nearly 8,000 children under age 5.
The nasal spray FluMist, the only flu vaccine made of live but weakened influenza virus, now is sold only for children 5 and older. Manufacturer MedImmune Inc., which funded the new research, plans to seek government approval to sell FluMist for younger children as well.
And children are the prime spreaders of flu virus, fueling infections in older people who may not survive. Each winter, flu kills 36,000 Americans, most of them elderly.
“Our current thinking is that to control influenza, we really have to vaccinate all children,” said Dr. Robert Belshe, a prominent vaccine specialist at St. Louis University who led the new study. “Anything that makes it easier and more effective (to vaccinate) children is going to contribute a lot to the protection against influenza.”
The study did find a safety concern: A few of the very youngest patients, those ages 6 months to 2 years, had an episode of asthma-like wheezing in the weeks after the first FluMist dose.
The increased risk was slight — 1 percent more children wheezed after FluMist than after flu shots — and the reaction was temporary. But Belshe still is analyzing whether the risk would offset the increased flu protection, and regulators undoubtedly will ask whether it means FluMist should be used only after age 2.
Loading...
As a matter of note FluMist has received approval for children and adults 2-49 years of age. FluMist should not be administered to children less than 24 months of age due to an increased risk of hospitalization and wheezing that was observed in clinical trials. FluMist should not be administered to any individual with asthma and to children under 5 years of age with recurrent wheezing unless the potential benefit outweighs the potential risk. Also FluMist should not be administered to individuals with severe asthma or active wheezing.
FluMist is contraindicated in individuals with history of hypersensitivity to eggs, egg proteins, gentamicin, gelatin or arginine or with life-threatening reactions to previous influenza vaccinations, and in children and adolescents receiving concomitant aspirin or aspirin-containing therapy.
Most common adverse reactions (occurring at ≥10% in individuals receiving FluMist and at least 5% greater than in placebo) are runny nose or nasal congestion in recipients of all ages, fever >100°F in children 2-6 years of age, and sore throat in adults.
FluMist is contraindicated in individuals with history of hypersensitivity to eggs, egg proteins, gentamicin, gelatin or arginine or with life-threatening reactions to previous influenza vaccinations, and in children and adolescents receiving concomitant aspirin or aspirin-containing therapy.
Most common adverse reactions (occurring at ≥10% in individuals receiving FluMist and at least 5% greater than in placebo) are runny nose or nasal congestion in recipients of all ages, fever >100°F in children 2-6 years of age, and sore throat in adults.
Loading...