Licensed Dietitian
588 posts
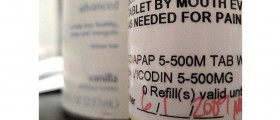
The FDA announced a recall of about 11 million bottles of store-brand acetaminophen 500-milligram caplets because small metal fragments had been found in a small number of these caplets. The metal fragments range in size from "microdots" to 8-millimeter pieces of wire and have been found through the regulatory quality-control procedures by the Perrigo Company of Allegan that makes the caplets.
Acetaminophen is an over-the-counter drugs for pain relief and fever reduction sold by Wal-Mart, CVS, and other drug stores, grocery stores, and wholesalers.
According to the FDA reports no illnesses, injuries, or consumer complaints have been reported so far and it is believed that the chances of serious adverse health consequences are small.
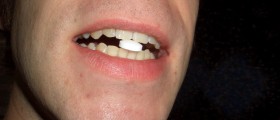
It is thought that if the caplets were swallowed, they could cause some stomach discomfort and possibly lead to cuts to the mouth or throat.
Consumers can find all the information regarding the companies that sell store-brand 500 milligram acetaminophen and the batches released at the FDA's web site at www.fda.gov/oc/po/firmrecalls/perrigo/perrigocustlist.html.
Consumers who realize that they have the recalled acetaminophen are advised to discontinue use immediately and contact Perrigo's consumer affairs department. Those who believe that they may be harmed by the drug should contact their doctors.
Perrigo and the FDA are working together on correcting the problem.
Acetaminophen is an over-the-counter drugs for pain relief and fever reduction sold by Wal-Mart, CVS, and other drug stores, grocery stores, and wholesalers.
According to the FDA reports no illnesses, injuries, or consumer complaints have been reported so far and it is believed that the chances of serious adverse health consequences are small.
It is thought that if the caplets were swallowed, they could cause some stomach discomfort and possibly lead to cuts to the mouth or throat.
Consumers can find all the information regarding the companies that sell store-brand 500 milligram acetaminophen and the batches released at the FDA's web site at www.fda.gov/oc/po/firmrecalls/perrigo/perrigocustlist.html.
Consumers who realize that they have the recalled acetaminophen are advised to discontinue use immediately and contact Perrigo's consumer affairs department. Those who believe that they may be harmed by the drug should contact their doctors.
Perrigo and the FDA are working together on correcting the problem.
Loading...