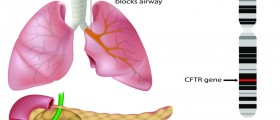
Cystic fibrosis is a genetic (inherited) chronic disease caused by a flaw in genes for the channels that allow salt to enter cells, causing the body to produce abnormal secretions, leading to a sticky buildup of mucus in the lungs, pancreas, and intestine. It affects the lungs and digestive system of about 30,000 children and adults in the United States and 70,000 people worldwide. Without treatment, CF results in death for 95 percent of affected children before age five. However, intensive treatment can prolong the lives of patients into their 30s and 40s.
Israeli researchers had tested a new, experimental drug that blocks the genetic flaw responsible for one form of cystic fibrosis and the results have been promising, they report.
People who participated in the Israeli trial had cystic fibrosis because of a mutation in the salt channel gene that produces a false "stop" signal, preventing the proteins forming the channel from being produced.
New Jersey biotechnology company, PTC Therapeutics, developed the drug through a screening program that singled out molecules that block such genetic "stop" signals. The screening program involved hundreds of thousands of compounds, with a light-emitting molecule called luciferase, which were aimed at the genetically altered "stop" signal. It was actually several compounds that hit the target and lit up but the scientists selected the best ones, modify them and came up with PTC124.
During the Israeli trial, 23 patients suffering from cystic fibrosis received the drug, in two cycles. In the first cycle, they had received three doses a day for 14 days and then, after a 14-day pause, three higher daily doses. The results were promising as the salt flow reached the normal range in 13 of the 23 cases in the first treatment cycle and in 9 of 21 cases in the second cycle.
The form of cystic fibrosis that the drug targeted is responsible for about half of all cystic fibrosis cases in Israel but for only about 10% of cases in the United States, where other genetic flaws in channel production predominate.
Since this was a short-term study without placebo, a longer-term trial needs to be done, probably early next year. The long term trial will be international, involving patients from the United States, Canada and Europe.
The drug is thought to be useful for a number of other genetic disorders caused by the same sort of mutation and is currently being tested for Duchenne muscular dystrophy, another genetic condition.
Israeli researchers had tested a new, experimental drug that blocks the genetic flaw responsible for one form of cystic fibrosis and the results have been promising, they report.
People who participated in the Israeli trial had cystic fibrosis because of a mutation in the salt channel gene that produces a false "stop" signal, preventing the proteins forming the channel from being produced.
New Jersey biotechnology company, PTC Therapeutics, developed the drug through a screening program that singled out molecules that block such genetic "stop" signals. The screening program involved hundreds of thousands of compounds, with a light-emitting molecule called luciferase, which were aimed at the genetically altered "stop" signal. It was actually several compounds that hit the target and lit up but the scientists selected the best ones, modify them and came up with PTC124.
During the Israeli trial, 23 patients suffering from cystic fibrosis received the drug, in two cycles. In the first cycle, they had received three doses a day for 14 days and then, after a 14-day pause, three higher daily doses. The results were promising as the salt flow reached the normal range in 13 of the 23 cases in the first treatment cycle and in 9 of 21 cases in the second cycle.
The form of cystic fibrosis that the drug targeted is responsible for about half of all cystic fibrosis cases in Israel but for only about 10% of cases in the United States, where other genetic flaws in channel production predominate.
Since this was a short-term study without placebo, a longer-term trial needs to be done, probably early next year. The long term trial will be international, involving patients from the United States, Canada and Europe.
The drug is thought to be useful for a number of other genetic disorders caused by the same sort of mutation and is currently being tested for Duchenne muscular dystrophy, another genetic condition.
Loading...