Loading...
Loading...
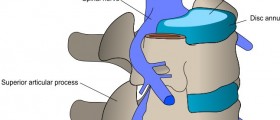
I just wanted to let you know that I also have a lot of trouble swollowing. When you look at my Exray, it shows the placement is somewhat forward and the cervicore disc rests on my Esophogus. When it gets inflamed or if i get a sore throat. OMG, I can feel it really bad. I have gotten used to it, thru the years, but sitll, I know that it is causing irreversible damage. Stryker, I do believe has covered ALL of our complaints and WE are all unfortunately Stuck with the consequences of their wrong doing. I would love to have mine out, but I know its too dangerous. I have TONS of problems Ranging from the swollowing issue to dysfunction of my right arm. I lose all control at times of it. Will drop anything that I am holding. Again. I know that there has been a lot of cover ups done and its up to all of us here to bust this wide open. We all need to ban together and go after them TOOTH AN NAIL.
Loading...
Loading...
Loading...
Well, it sounds like there is more to the failed or improper placements of Styker disc. I've complained to Stryker and their lawyers when I sent certified letters to all the board members. Not one board member responded to me. (ond member is a Stryker family with a last name of Stryker).....She is listed on their web page, Board Member')
Mine (fyi) was a failed c-6 c-7 in 2006. I have all the symtoms as many of you. They scheduled an MRI after many Myelograms in 2011. The original surgeon finally saw me after 5yrs, and ordered the MRI with concern of his partners work faulty.
Stryker had me sign another form (adendum) to keep me the patient in the program for 9 yrs in lieu of 3. But they would not pay for the MRI and I lost my insurance. I've been dumped and the doctors office say the FDA (federal) gave Stryker permission to drop this patient from the clinical trial with no responsibility whatsoever.
I'm screwed as most attorney's have told me as we all are at this time. I appreciate all pushing on a movement that has hurt and pained us for life an no recourse!
If anybody knows of a class action suit going yet, please advise me immediately, please......Thanks Dave
Loading...
Loading...
David Dowling wrote:
Well, it sounds like there is more to the failed or improper placements of Styker disc. I've complained to Stryker and their lawyers when I sent certified letters to all the board members. Not one board member responded to me. (ond member is a Stryker family with a last name of Stryker).....She is listed on their web page, Board Member')
Mine (fyi) was a failed c-6 c-7 in 2006. I have all the symtoms as many of you. They scheduled an MRI after many Myelograms in 2011. The original surgeon finally saw me after 5yrs, and ordered the MRI with concern of his partners work faulty.
Stryker had me sign another form (adendum) to keep me the patient in the program for 9 yrs in lieu of 3. But they would not pay for the MRI and I lost my insurance. I've been dumped and the doctors office say the FDA (federal) gave Stryker permission to drop this patient from the clinical trial with no responsibility whatsoever.
I'm screwed as most attorney's have told me as we all are at this time. I appreciate all pushing on a movement that has hurt and pained us for life an no recourse!
If anybody knows of a class action suit going yet, please advise me immediately, please......Thanks Dave
Please contact me Philomena.55
David, It is imperative that you contact Philomena.55 or me at _[removed]_. We know what you are going through right down to the litagation. Please contact us so we can fight Stryker and their evil surgeons. Looking forward to hearing from you.
Loading...
Loading...
Did your surgeon disclose to you that there was nickel in the CerviCore device? Were you given a metal prescreening test for all of the metals in the disc as required by FDA protocol for metal on metal spinal systems prior to implanting the disc? Did you submit to blood tests for HIV, all Hepatitises, and immune diseases (RF)? If not, then you surgeon did NOT FOLLOW the trial protocol if the trial was legitimate at the time your device was implanted.
The CerviCore Disc had been submitted to the FDA Black Box on December 21, 2007. This is the government database where "trial devices that are NOT APPROVED or cleared by US FDA" are required to be registered if they are NOT APPROVED FOR ANY USE. A little history on the CerviCore trial might help you understand the dilemma that we CerviCore victims are now trapped in. The trial was given a "conditional approval" IDE. This means that the sponsor, Stryker Spine had 45 days to implement the "conditions" - also, if the conditions related to the consent form, it was mandatory that compliance be immediate otherwise, the IDE became invalid. We will never know what these conditions were until we can get our hands on the Master File. But the fact that there were so many violations of protocol and the study ended up in the black box for "trial of device not approved or cleared by the US FDA" under a delayed posting calls into question the legitimacy of the entire trial. All surgeries after 2007 were definitely unauthorized implants.
I would like to have a meet up conference with all CerviCore subjects who would like to be proactive. My plan is for us to report our ADVERSE EVENTS TO THE FDA MAUDE DATABASE. This is what this database is for. All you have to do is google FDA MAUDE and you will have the link to the form. We should all discuss this at a meet up on my forum SpineTalk. I will email everyone a link and this will activate a private account on the forum. We can talk in private there in PMs about our strategy for exposing this clinical trial charade that has permanently injured our spines as well as caused a toxic reation to the nickel from metal poisoning. My point is, we need to get seriously organized otherwise we will suffer and die without any medical care or concern. This is a scandal for Stryker and no one is going to want to get tainted by this -- so we will be ignored by physicians. Therefore, our only recourse is to bring this to the attention of the proper authorities and expose Stryker for its biomedical deception and medical malfeasance. Our human rights were violated in ways that only happened in Nazi Germany -- and this must STOP.
I can be reached at Philomena.55 at gmail dot com. Or just email me in private.
What is Medical Device Reporting?
- The Medical Device Reporting (MDR) regulations require manufacturers who have received complaints of device malfunctions, serious injuries or deaths associated with medical devices to notify FDA of the incident.
- MDR regulations require User Facilities (e.g., hospitals, laboratories) to report suspected medical device related deaths to both the FDA and the manufacturers. User facilities must report medical device related serious injuries to the manufacturer.
See also:
Loading...
Loading...