***this post is edited by moderator *** *** private e-mails not allowed*** Please read our Terms of Use
Loading...
Am_burdock, you will need to register so that we can contact you in a PM. We need to get more information from you as soon as possible.
***this post is edited by moderator *** *** private e-mails not allowed***Please read our Terms of Use
Loading...
am_burdick wrote:
philomena.55 wrote:
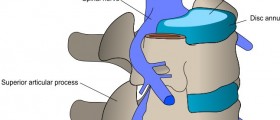
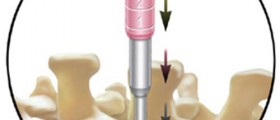
Hello - Has anyone on this board been a subject in a clinical trial for Stryker Spine Cervicore Interverterbral Disc - one level total disc replacement? I am looking for other subjects who were recruited into this trial that was sponsored by Stryker Spine for their IDE clinical trial of the CerviCore disc implant. I was a subject in 2008 and have had substantial adverse events that the PI has never reported to the sponsor or FDA. My cervical condition is worse post surgery and the neck - bilateral arm pain is unbearable. I have had a post surgery MRI, EMG and Myelogram CTscan that I had to pay for out of pocket that shows foraminal stenosis (bilateral) at the implanted disc site (C5-C6) and foraminal stenosis at adjacent disc, C4-C5 - right side, multi-level let side facet joint arthrosis and uncovertebral disease, reversal of lordosis - kyphosis, multi-level degenerative discs, possible left greater than right foraminal narrowing at C6-7 & left sided hypertrophy at C7-T1 and something showed up on the mylegram that said that the nerve roots at the disc implant site are shortened and blunted at the nerve root sleeve.
The PI who recruited me into this clinical trial refused to listen to my early onset of complaints (within 6 to 7 months) post surgery. He told me that my complaints were due to "old age" and refused to do any testing (as per the consent form) for post surgery complications, other than to take Xrays and state that the "prothesis was in position". I had to go to a neurosurgeon to get presriptions for the MRI/EMG and my pain clinic physician finally decided to order the myelogram/CTscan after a year of my chronic complaints about neck and arm pain that has rendered me totally disabled.
I saw that there was another member on this board who was in phase one of the trial (I was told that I was in phase two). The member goes by "painstaken" and I was wondering if this member is still on this board -- as I think we need to compare notes.
The consent form that I signed does not mention anything about the risk of foraminal stenosis or reversal of lordosis-kyphosis complications post surgery. The neurosurgeon who ordered the MRI etc said that I had to return to the PI (prinicple investigator) and show him the film she copied that show the adjacent disc stenosis. When I returned to the PI - he denied the existence of stenosis - and did not report anything to the sponsor or FDA. My MRI prior to the surgery showed only a herniated disc w/ bilateral stenosis at C5-C6. The PI said I had to have a myelogram to collaborate the MRI but refused to order it and have Stryker pay for it pursuant to the terms of the consent form.
I finally wrote a letter to Stryker, the IRB and the PI and his research nurse, and have heard nothing back from them. I had to contact the FDA and file a complaint to initiate an investigation as to why the priniciple investigator is not complying with the FDA and reporting unaticipated and adverse events post surgery.
If anyone has any answers or can tell me what I am supposed to do to get this thing removed from my neck, have the two or three teir fusion and correct the reversal of lordosis-kyphosis please let me know as soon as you read this. I cannot get another neuro or orthopaedic surgeon to look into this as they do NOT WANT TO GET INVOLVED in another surgeon's clinical trial mess up. My patient rights as a clinical trial subject have been criminally violated and I have no idea if I am the only subject of this clinical trial that was abused by the PI or if this is a pattern with all of the Stryker spine PIs who willfully refuse to investigate post surgery complaints of the subjects and report the adverse events/complications to the sponsor and FDA as is required by the CFR regulations for high risk medical device IDE surgeries.
I too had the replacement, and it has made everything worse. They won't remove it because of an artery runs in front and if they nick it you cAn bleed to death. I had mine fused with the disc remaining in...
Am_burdock, you will need to register so that we can contact you in a PM. We need to get more information from you as soon as possible. I have a gmail account. Please contact me there using my screen here.
I too had the cervicore implant in my back. It failed. I had to have a third surgery to have it removed and my back fused. Stryker paid for this surgery. I still have daily pain.
Loading...
Loading...
I have corresponded recently with Philomania55. I have posted here previously, but wanted to update. My pain is getting worse as time goes by. I am experiencing a lot of very perilous rash issues that pop up and take a long time to heal. They leave some pretty nasty scars when they do finally heal up. I shared a photo of one of these with Philomania 55 . She can share it with anyone who is having any similar experiences. I am curious if this is a common side effect of this procedure.
The doctor who did the surgery has had me back and paid me for the last five years to get blood tests and X-rays done. He keeps telling me what a success the study was, and that Stryker just stopped trying to get approval for the implant because there were so many similar ones on the market that it was not economical for them to pursue final FDA approval. Now they want me to come back for another three years to continue the bloodwork and X-rays. This seems odd in that they claim they are dropping the product and not continuing with the process to get approval. I feel quite deceived in this process.
My new Dr.is a very kind and understanding Physician who has actually requested my records and surgery info three for four different times from the Dr who did the surgery. He has never sent it to her. This seems criminal. I have signed all of the necessary forms for him to release the records. When I went and asked for them personally, I was told that it would take a long time for someone to copy all the documents and to come back another time to get them. When I went back to get them , they claimed they had been sent back to Stryker as part of the study, and that they did not have a McCoy to give me. Long story short, all I ever got was copies of the X-rays from the follow out visits.
I would like to know if anyone else has experienced these types of issues. Please respond and let me know.
Loading...
You know this is very interesting. By that and after the last 6 yrs, I beleive this Clinical Trial has many a secrets that we'll never have full disclosure. I too, saw my file with the surgeon. Many of my records have been removed. The surgeons nurse has even told me she is very upset with the surgeon. My case file is so huge I can't even begin to share with you and others without sitting down for hours as to what all happened to me, and it is negligence, malpractice, but mostly cruel in fact. I don't know if you or others have read my previous replies or not. I would like an idea of just how many subjects or victims there are. I keep reading these cases sent to me, but is there anything that can be done? After two major surgeries with mylograms, xrays, speech therapist, swallowing problems etc etc, I am worse off then ever. Yes I have had an outbreak just recently of a terrible rash. I went to my GP and he was concerned. After some antibiotics and cream etc, it finally healed. Small rashes have occurred consequently. There is so much to detail that I can't do it all in a small post. Specifically the surgery was a botch from day one. I was told on the second surgery I had, they were to remove the implant at c-6 c- 7 and major fusion in lieu. Then I found out that they didn't remove the failed disc, I don't beleive any words coming from the surgeons. I was stunned when I looked in the mirror. First surgery anterior with small scar. The second one left me with a gross chunk of my neck posteriorly gone...This included removal of muscle bone etc. I am embarrassed when people ask me, "My God, what happened to you". Lastly, I have a large law firm investigating my case. They are already working on a case against Stryker. It is being avertised on TV channels. I personally and honestly beleive the surgeons are protected by our agreement with Stryker. Had I carefully read the agreement and know what I know now, the surgery would have never taken place. But when you are in severe pain and beleive the Stryker rep, you'll do about anythiing to be free of pain and misery. I was also told by the Stryker rep post second surgery, that most of the patients had absolutely no adverse problem issues. So she was indicating that my failed disc was unique and near and a isolated case! So there are lies from bottom to top. Thank you for keeping me in the loop. Don't take this personal, but I am comforted knowing there are others suffering with me. By that I mean I was the only one in the entire world with this issue of a Stryker failed disc. DD
Loading...
To all CerviCore victims! This may come as a surprize to you, but the CerviCore Disc was NEVER APPROVED OR CLEARED FOR ANY USE BY THE FDA. What this means is that the device that was implanted in your cervical (not back, Rhonda!) spine was unauthorized and illegal. We were all duped by Stryker and the surgeons to participate in a secret covert study that was never fully approved for an IDE. I have all the proof of this and all you need to do is google - Trial of Device NOT APPROVED OR CLEARED by US FDA and NCT00588601.
All of the information is in that database and I can show you how to decode FDA definitions. The most important definition is that the CerviCore device was ordered registered by Public Law on December 21, 2007 under a delayed posting.
The FDA definition of "DELAYED POSTING" is Delayed Posting? (FDAAA) Definition: If this is a Section 801 applicable clinical trial, indicate whether this trial includes a device NOT previously approved or cleared by the US FDA for any use, as specified in US Public Law 110-85, Title VIII, Section 801. Select Yes/No. If "Yes" is selected, full posting of the trial information on ClinicalTrials.gov will be delayed until after the device has been approved or cleared.
You will have to uncheck all of the links to get to the gray page - if you need any assistance in decoding the information in the FDA archives for "Trial of device NOT APPROVED OR CLEARED by US FDA and entered as a delayed posting
***this post is edited by moderator *** *** private e-mails not allowed***Please read our Terms of Use
Time is of the essence - we need to find out how many of us were injured and help you get assistance (not from Stryker - they will screw you over again as this is a huge criminal cover up).
All of the surgeons knew that Stryker never received a valid ORDER FOR AN IDE for the actual device that was implanted. Plus, they only were given a conditional approval by the IRB -- which approved the corporate ID protocol CT-002-04. Look for that ID number on your consent forms. It will match up with all of the information in the FDA black box for trial of device not approved or cleared by US FDA - under delayed posting.
If anyone from Stryker contacts you now that the study has been ABRUPTLY SHUT DOWN by the FDA -- contact an attorney immediately. They will attempt to buy your silence for $10,000.00 -- and that won't even cover the first year of medical expenses and pain management that you will need for the remainder of your life -- given that they never told us about the nickel in the CerviCore device or that they were contaminated and never approved by the FDA for any use what so ever.
Philomena.55
Loading...
philomena.55 wrote:
am_burdick wrote:
philomena.55 wrote:
Hello - Has anyone on this board been a subject in a clinical trial for Stryker Spine Cervicore Interverterbral Disc - one level total disc replacement? I am looking for other subjects who were recruited into this trial that was sponsored by Stryker Spine for their IDE clinical trial of the CerviCore disc implant. I was a subject in 2008 and have had substantial adverse events that the PI has never reported to the sponsor or FDA. My cervical condition is worse post surgery and the neck - bilateral arm pain is unbearable. I have had a post surgery MRI, EMG and Myelogram CTscan that I had to pay for out of pocket that shows foraminal stenosis (bilateral) at the implanted disc site (C5-C6) and foraminal stenosis at adjacent disc, C4-C5 - right side, multi-level let side facet joint arthrosis and uncovertebral disease, reversal of lordosis - kyphosis, multi-level degenerative discs, possible left greater than right foraminal narrowing at C6-7 & left sided hypertrophy at C7-T1 and something showed up on the mylegram that said that the nerve roots at the disc implant site are shortened and blunted at the nerve root sleeve.
The PI who recruited me into this clinical trial refused to listen to my early onset of complaints (within 6 to 7 months) post surgery. He told me that my complaints were due to "old age" and refused to do any testing (as per the consent form) for post surgery complications, other than to take Xrays and state that the "prothesis was in position". I had to go to a neurosurgeon to get presriptions for the MRI/EMG and my pain clinic physician finally decided to order the myelogram/CTscan after a year of my chronic complaints about neck and arm pain that has rendered me totally disabled.
I saw that there was another member on this board who was in phase one of the trial (I was told that I was in phase two). The member goes by "painstaken" and I was wondering if this member is still on this board -- as I think we need to compare notes.
The consent form that I signed does not mention anything about the risk of foraminal stenosis or reversal of lordosis-kyphosis complications post surgery. The neurosurgeon who ordered the MRI etc said that I had to return to the PI (prinicple investigator) and show him the film she copied that show the adjacent disc stenosis. When I returned to the PI - he denied the existence of stenosis - and did not report anything to the sponsor or FDA. My MRI prior to the surgery showed only a herniated disc w/ bilateral stenosis at C5-C6. The PI said I had to have a myelogram to collaborate the MRI but refused to order it and have Stryker pay for it pursuant to the terms of the consent form.
I finally wrote a letter to Stryker, the IRB and the PI and his research nurse, and have heard nothing back from them. I had to contact the FDA and file a complaint to initiate an investigation as to why the priniciple investigator is not complying with the FDA and reporting unaticipated and adverse events post surgery.
If anyone has any answers or can tell me what I am supposed to do to get this thing removed from my neck, have the two or three teir fusion and correct the reversal of lordosis-kyphosis please let me know as soon as you read this. I cannot get another neuro or orthopaedic surgeon to look into this as they do NOT WANT TO GET INVOLVED in another surgeon's clinical trial mess up. My patient rights as a clinical trial subject have been criminally violated and I have no idea if I am the only subject of this clinical trial that was abused by the PI or if this is a pattern with all of the Stryker spine PIs who willfully refuse to investigate post surgery complaints of the subjects and report the adverse events/complications to the sponsor and FDA as is required by the CFR regulations for high risk medical device IDE surgeries.
I too had the replacement, and it has made everything worse. They won't remove it because of an artery runs in front and if they nick it you cAn bleed to death. I had mine fused with the disc remaining in...
Am_burdock, you will need to register so that we can contact you in a PM. We need to get more information from you as soon as possible. I have a gmail account. Please contact me there using my screen here.I too had the cervicore implant in my back. It failed. I had to have a third surgery to have it removed and my back fused. Stryker paid for this surgery. I still have daily pain.
Rhonda,
Tell us more about your back surgery with CerviCore.
Philomena.55
Loading...
Hello, I too had a CerviCore replacement back in 2008. I would like some more information. I have continued to have problems but my surgeon keeps saying my neck looks fine sayes I just need more PT. I have had to quit work because of the pain I just had new MRI's done 3 days ago that were ordered by my Rheumatologist. I know I need to wait and see the results. But I always wondered if there was a problem with the disk.
Loading...
Dave, please contact me as soon as you get this - OK. I am attempting to interest one of the top clinical trial and bioethics law firms into investigating CerviCore
Loading...
Wow a clinical trial you say, well i am in vancouver canada and not only did i have 1 but 2 levels put in as regular surgery in 2008 no one said anything about clinical trials and all the issues you are having, got every one one of them, plus Osteo Arthritis, a local prominant Nuerologist diagnosed me with Multifocal Myofascial pain = fibromyalgia or who knows, 100 different opinions
***this post is edited by moderator *** *** private e-mails not allowed*** Please read our Terms of Use
Loading...
I just had surgery 11/5/13 to have my implant removed. At the time of my first surgery c5-6 was questionable but i was recruited into this study group with the promise of better mobility and less stress on adjacent levels. I had my cervicore implant inserted 11/06. I started having pain after about a year. I related this to my physician at each follow up appointment and was told that the sight of the disc looked fine although because of the metal in the device achieving a clear view was impossible with an MRI. After years of suffering and several painful injections I finally after seven years had the surgery to remove the device and fuse c56 and c67. My surgeon told me that the device was covered in what he described as gunk. Apparently my body was rejecting the implant for some time. And was undetectable by ct scans and MRI's. I'm feeling overwhelmed at what was happening to my body all these years and what I may be left with permanently as a result of this device' I have not found any information on the FDA website. Any information that I could get from others with similar experiences would be greatly appreciated.
Loading...
Hello need more info -- my name is Philomena. I would love to talk to you in private. I will get back to you as soon as possible. please try to contact me as soon as possible.
***this post is edited by moderator *** *** private e-mails not allowed*** Please read our Terms of Use
Loading...
Need more info, if you do not register on this forum we will not be able to exchange information in private email. Otherwise the moderator delete anything that helps us stay in touch.
Loading...
Needmoreinfo, several of us CerviCore victims are very interested in hearing from you again. We can answer some of your questions and we would like to ask you some questions about how you were able to finally find a surgeon who would remove the device. Did Stryker pay for the revision surgery? Did the surgeon send a report back to FDA?
Loading...