The PI who recruited me into this clinical trial refused to listen to my early onset of complaints (within 6 to 7 months) post surgery. He told me that my complaints were due to "old age" and refused to do any testing (as per the consent form) for post surgery complications, other than to take Xrays and state that the "prothesis was in position". I had to go to a neurosurgeon to get presriptions for the MRI/EMG and my pain clinic physician finally decided to order the myelogram/CTscan after a year of my chronic complaints about neck and arm pain that has rendered me totally disabled.
I saw that there was another member on this board who was in phase one of the trial (I was told that I was in phase two). The member goes by "painstaken" and I was wondering if this member is still on this board -- as I think we need to compare notes.
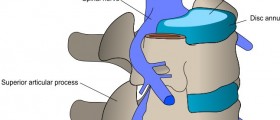
The consent form that I signed does not mention anything about the risk of foraminal stenosis or reversal of lordosis-kyphosis complications post surgery. The neurosurgeon who ordered the MRI etc said that I had to return to the PI (prinicple investigator) and show him the film she copied that show the adjacent disc stenosis. When I returned to the PI - he denied the existence of stenosis - and did not report anything to the sponsor or FDA. My MRI prior to the surgery showed only a herniated disc w/ bilateral stenosis at C5-C6. The PI said I had to have a myelogram to collaborate the MRI but refused to order it and have Stryker pay for it pursuant to the terms of the consent form.
I finally wrote a letter to Stryker, the IRB and the PI and his research nurse, and have heard nothing back from them. I had to contact the FDA and file a complaint to initiate an investigation as to why the priniciple investigator is not complying with the FDA and reporting unaticipated and adverse events post surgery.
If anyone has any answers or can tell me what I am supposed to do to get this thing removed from my neck, have the two or three teir fusion and correct the reversal of lordosis-kyphosis please let me know as soon as you read this. I cannot get another neuro or orthopaedic surgeon to look into this as they do NOT WANT TO GET INVOLVED in another surgeon's clinical trial mess up. My patient rights as a clinical trial subject have been criminally violated and I have no idea if I am the only subject of this clinical trial that was abused by the PI or if this is a pattern with all of the Stryker spine PIs who willfully refuse to investigate post surgery complaints of the subjects and report the adverse events/complications to the sponsor and FDA as is required by the CFR regulations for high risk medical device IDE surgeries.
Loading...
This is a correction by the author. I realized that I made a mistake regarding the pre surgery MRI. I had left side moderate to severe stenoses prior to the surgery. The recent myelogram and CT scan now indicates that I have bilateral stenosis at C5-C6 with nerve sleeve root blunting and shortening. Hence, the stenosis was never adequately treated before the implantation and now the stenosis has either returned or was never removed before replacing the cervicore disk.
Loading...
Sorry about your pain. Yes, cervicore has ruined my life also. I wish I could warn the world about the evil disc and the butchers that implant them. I begged for two years for them to remove it. They called me "over dramatic" because I"m a woman and I was in so much pain and couldn't swallow. I felt like I was being strangled. When they removed it, they found that scar tissue had formed and was pressing on the esophogus, trachea and spinal cord. Of course, they minimized this information. They didn't tell me that they also had to pry cervicore out and in doing so took out 3/4 of my upper c5 and c6, so the 2nd surgery fusion failed!!!
I wish I had any advice but due to the extensive damage from the cervicore, the 3rd surgery failed as well because my vertabrae can not
withold any weight. I was 37 and a track coach before this. I can barely get through the day and have not run in over 3 years.
I understand your frustration, I went through every surgeon that would see me in Pittsburgh and they all said they couldn't (wouldn't ) help me. My only advice is go to a renowned clinic near you and get a complex cervical reconstruction orthopedic surgeon.. I am now at cleveland clinc in ohio and my 4th surgery in Nov. 1st. He was appalled at the damage from one disc.. but cant find an attorney to take on stryker or the famous surgeon that implanted it and played God...
Please keep me updated.. It is so unfair to suffer at the hands of arrogant surgeons and scrupulous pharm. corporations... Good Luck - Melinda
Loading...
We have a lot to discuss. I know of other CerviCore failers. Are you in Pittsburgh? My husband's company is HQ'd there. I have some answers for you and we may be able to get something done legal wise. I have already contacted the FDA and there is an investigation into the clinical trial in place. Please let me know if you get this and I will try and find your direct email addy to get back with you. It is very important that we speak as soon as possible. It sounds as though you have had serious adverse events and I am wondering if your PI reported them to the FDA in a timely manner. I have tons of research that you may be interested in.
***this post is edited by moderator *** *** private e-mails not allowed **
Please read our Terms of Use
Go here and sign up - I am one of the moderaters.***edited by moderator*** web addresses not allowed My contact there is PAG.
Loading...
Yes, I am from Pittsburgh and I look forward to talking with you. I have to be up extremely early - struggling through teaching with tons of steroids in order to keep my med. benefits. Please email me and let me know when we can set up a time to talk. I'm usually home by 4.
My email is ***this post is edited by moderator *** *** private e-mails not allowed **
Please read our Terms of Use and my phone is *** private info removed ***. The last 3 and a half years have been a isolated nightmare, I hope we can find some answers.. Talk to you soon. Melinda
Loading...
Loading...
My PI should have informed your PI about the findings in my Myelogram of the 8 tumors? I am shocked that he did not do this and allowed others to be implanted with the disc knowing that it caused tumors around the artificial disc. What state are you in? You need to consult an attorney - as you still may be able to get the competent medical attention you need before your statutes of limitations run on medical negligence by your surgeon. There is a lot of imformation out bout the dangers of these metal on metal devices causing metallosis and osteolysis -- and I know that Stryker surgeons are NOT going to tell you what is going on based on my and several other CerviCore subjects that I have spoken to at length. Stryker has a duty to protect your health and welfare and you have a lot to lose if you do not find out as soon as possible what is going on with your disc. I do not want to suggest any symptoms so I will not go into any specifics about mine now - but if you email me I will send you my images that you can show your surgeon and this might instigate your surgeon to order tests - i.e. myelogram and bone scan to make certain that you are not at risk for metallosis from the nickel in the disc. And you may still have time to get an attorney to enforce the terms of your consent form to make sure you don't have to pay for the imagining tests. I had to pay for mine -- as the PI I had would flat out not listen to my complaints -- but now that I know he expected to make millions from royalties after the disc was on the market -- it makes perfect sense - Greed outweighed his adhering to the ethical contract he signed with the FDA called an investigators agreement to protect your health and welfare first and foremost. Did you know that there are 3 different versions of the Cervicore disc on the internet? I have no idea which one was officially approved by the FDA for the IDE.
Loading...
Loading...
Loading...
---I too am a Stryker implant victim. My implant was installed inDecember of 2006. My symptoms got worse and worse. I complained for nearly 3 years to the PI. He would tell me each time that I was OK and to continue PT.
I knew I was worse than before going in for an implant. I resorted to paying for another neurosurgeon that told me I was in perilous condition. I sent that dictation same day courier to my surgeon. Then and only then did he do another surgery and now I've the same symptoms but worse.
So far I cannot find aan attorney to take the case because of the statute of limitations. I sent letters to all the board members of Stryker and heard nothing back from them. I also lost my insurance through a divorce and I paid out of pocket for a myelogram all meds and some PT.
I am incapable of working and turning 58. I feel I was a victim of mal practice in an array of ways. Currently a law firm is reviewing my case and my file is large. We'll see.
How does one contact the FDA ?
Thanks ,Dave
Loading...
Looking forward to talking to u and I do have a complaint into the FDA and I will help you do the same. I have a lot of information and there is a group formed on facebook as well --
***this post is edited by moderator *** *** private e-mails not allowed*** Please read our Terms of Use
Loading...
Loading...
Loading...