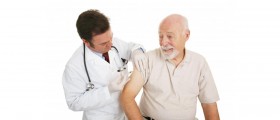
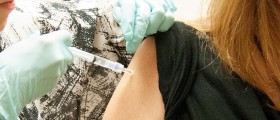
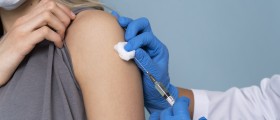
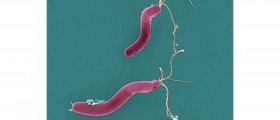
Thousands of doses of the meningitis C vaccine have been withdrawn by the manufacturer Novartis due to fears of contamination.
Such move was taken after samples from two batches found presence of a bacterium.
The meningitis C vaccine is one of the routine immunisations in childhood, with the first doses normally given when a child is 3 and 4 months old.
The government's medicines agency said contaminated vaccine had not been distributed to the UK and the move was "entirely precautionary". There should be no concern that the UK children may be exposed to the risk.
Novartis reported being "committed to being a safe and reliable provider". Around 21,000 doses in total have been removed from the shelves. The recalled batches of the Menjugate Kit, which were manufactured in Italy, had passed safety checks before leaving the factory to be transported by road to the UK. However, a small number of samples transported to another country by air, were later found to be contaminated with staphylococcus aureus bacteria.
A spokeswoman for the Medicines and Healthcare products Regulatory Agency reported that the samples that failed the sterility test were part of a non-routine study undertaken by the company and were not part of the UK market product. The move was a precautionary none of the samples that had come to the UK had been contaminated. The products that have been used in the UK had passed all the necessary quality standards and are perfectly safe.
The Department of Health reiterated that the recall was a precautionary measure and added that anyone who was concerned after taking the vaccine should contact their GP or NHS Direct.
Such move was taken after samples from two batches found presence of a bacterium.
The meningitis C vaccine is one of the routine immunisations in childhood, with the first doses normally given when a child is 3 and 4 months old.
The government's medicines agency said contaminated vaccine had not been distributed to the UK and the move was "entirely precautionary". There should be no concern that the UK children may be exposed to the risk.
Novartis reported being "committed to being a safe and reliable provider". Around 21,000 doses in total have been removed from the shelves. The recalled batches of the Menjugate Kit, which were manufactured in Italy, had passed safety checks before leaving the factory to be transported by road to the UK. However, a small number of samples transported to another country by air, were later found to be contaminated with staphylococcus aureus bacteria.
A spokeswoman for the Medicines and Healthcare products Regulatory Agency reported that the samples that failed the sterility test were part of a non-routine study undertaken by the company and were not part of the UK market product. The move was a precautionary none of the samples that had come to the UK had been contaminated. The products that have been used in the UK had passed all the necessary quality standards and are perfectly safe.
The Department of Health reiterated that the recall was a precautionary measure and added that anyone who was concerned after taking the vaccine should contact their GP or NHS Direct.
Loading...