
Cyanide
Just half a gram (less than 1/50 of an ounce) of sodium cyanide is fatal if taken internally, and just a gram (about 1/30 of an ounce) is fatal if in contact with the skin. This best known of all poisons is even found in several food plants, in which it serves to deter predators. Cyanide is a component of the pits and seeds of stone fruits, such as apricots, peaches, and apples. It is also found in toxic concentrations is cassava root, which has to be throughly processed before it is transformed into edible manioc, a staple food in much of the tropics, and tapioca. The popular sweetener aspartame (Nutrasweet) actually does break down into cyanide when it is digested, but in very, very small amounts.
- Important notification about information and brand names used in this slideshow!
- Photo courtesy of Julo by Wikimedia Commons : en.wikipedia.org/wiki/File:Kaliumcyanid.jpg
- io9.com/5861680/10-of-the-most-dangerous-chemicals-in-the-world
- http://beforeitsnews.com/alternative/2013/01/top-10-most-deadly-poisons-known-to-mankind-2526172.html
- http://articles.mercola.com/sites/articles/archive/2006/08/08/the-best-deadly-poisons-ingested-or-inhaled.aspx

Arsenic
Thousands of murder mystery feature killing with arsenic. A poison that can build up in the body over a long time, arsenic can be used criminally as an illicit additive to food and drink, but it can also accumulate in the body from contact with contaminated water, contaminated soil, and lumber dyed green with arsenic used in housing and even in playsets. A lethal dose of arsenic in an adult is typically about 1 gram for every 10 kilograms of body weight, or 1/4 of an ounce for an average-sized adult. However, inorganic arsenates from chemicals are much more toxic than arsenic compounds found in plant foods; some nutritionists even believe that the body needs tiny amounts of arsenic for normal function. If you are ever poisoned by arsenic, it is most likely to be from your drinking water, and the first signs of the problem will be the appearance of unusual lines on your fingernails.
- Important notification about information and brand names used in this slideshow!
- Photo courtesy of adplayers by Flickr : www.flickr.com/photos/adplayers/9672316071/
- Smith AH, Hopenhayn-Rich C, Bates MN, et al. (July 1992). "Cancer risks from arsenic in drinking water". Environ. Health Perspect. 97: 259–67.
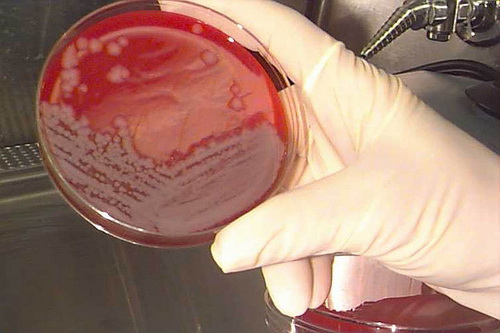
Anthrax
Strictly speaking, anthrax is a pathogen, not a toxin, since it is a bacterium, but its powdery spores and its use in terror attacks cause it to be associated with other common poisons. Anthrax reproduces itself by spores, which in nature are carried through the air and cause disease when they come in contact with the skin or lungs. When the spores are breathed in, they cause flu-like symptoms that may quickly progress to pneumonia and death. When they come in contact with the skin, they cause cutaneous lesions that are usually not fatal. It takes 10 to 20 thousand spores breathed into the lungs to make lethal dose, just a tiny fraction of a gram. Bodies of people and animals that have died of the disease become a source of infection.
- Important notification about information and brand names used in this slideshow!
- Photo courtesy of U.S. Army Medical Research Institute of Infectious Diseases photo by Wikimedia Commons : commons.wikimedia.org/wiki/File:Anthrax_culture.jpg
- Bravata DM, Holty JE, Liu H, McDonald KM, Olshen RA, Owens DK (February 2006). "Systematic review: a century of inhalational anthrax cases from 1900 to 2005". Ann Intern Med 144 (4): 270–80.

Mercury
Mercury poisoning is surprisingly common in Japan and other countries where whales and dolphins are sometimes eaten. Marine mammals accumulate mercury by eating fish that in turn eat smaller fish that eat sea plants that absorb mercury contamination. Mercury poisoning also sometimes occurs in people who work in plants that produce electrical equipment and crematoria. Mercury reacts with the antioxidant selenium, making it unavailable for the creation of enzymes that are essential for the health of neurons. Long-term mercury poisoning usually causes neuropathy, a painful condition of nerve damage in the hands and feet, and can be linked to delays or failures of mental development.
- Important notification about information and brand names used in this slideshow!
- Photo courtesy of Bionerd by Wikimedia Commons : en.wikipedia.org/wiki/File:Pouring_liquid_mercury_bionerd.jpg
- Clifton JC 2nd (2007). "Mercury exposure and public health". Pediatr Clin North Am 54 (2): 237–69, viii.

Sulfur Mustard
The sulfur mustards, better known as mustard gas, are invisible gases that cause severe blistering when they come in contact with the skin or respiratory tract. People exposed to the gas seldom experience immediate symptoms, but 24 hours later they develop painful, swollen blisters where the gas has come in contact with tissue. On the skin, mustard gas causes only "light" first or second degree burns, but because of the blistering, they can become as disfiguring as third degree burns. The burns can be prevented if affected tissues are treated immediately with a simply povidone-iodine spray, but because the chemical usually is not detected until it begins to destroy tissue effective treatment is rare. Often mustard gas burns cannot be bandaged or touched without causing excruciating pain. Mustard gases were used in the 1920's as the very first chemotherapy for cancer; they are still used in modified form for some kinds of cancer treatment today.
- Important notification about information and brand names used in this slideshow!
- Photo courtesy of US Government by Wikimedia Commons : en.wikipedia.org/wiki/File:155mmMustardGasShells.jpg
- Stewart, Charles D. (2006). Weapons of mass casualties and terrorism response handbook. Boston: Jones and Bartlett. p. 47.

Digitalis
Digitalis, the herb derivative which is also used in the common heart medication Digoxin (most commonly marketed under the trade name Lanoxin in the United States and Canada), can both heal and harm. As a medication, Digoxin, now synthesized, but once used a purified chemical extract from the foxglove plant, makes the heart beat more forcefully. It can stop "flutter" and atrial fibrillation. Even when used as a medication, however, digoxin has a very narrow therapeutic range. Too much can cause the heart to beat uncontrollably, with extra heart beats, headache, psychosis, delirium, and even heart attack and death. The side effects of digoxin (Lanoxin) are aggravated when excessive use of diuretics ("pee pills") like Lasix cause low potassium levels, and the drug usually cannot be taken with the heart medications amiodarone or verapamil, or with certain antibiotics, such as erythromycin. First symptoms of a toxic reation can include "seeing yellow," but any sudden and severe changes in heart capacity or muscle tone are a reason to call the doctor.
- Important notification about information and brand names used in this slideshow!
- Photo courtesy of Daniel Parks by Flickr : www.flickr.com/photos/parksdh/5764119095/
- Goldfrank LW (2006). Goldfrank's Toxicologic Emergencies (8th ed.). New York: McGraw-Hill.

Hydrogen Peroxide
Hydrogen peroxide isn't exactly a deadly poison. Our own bodies make hydrogen peroxide in minute amounts, quickly neutralized by antioxidants, and it is safe to put small amounts of the substance diluted in lots of water in your mouth for a rinse you spit out. Swallowing even dilute hydrogen peroxide, however, is not a good idea. The concentrated hydrogen peroxide often sold by natural health stores can erode the lining of the throat, the stomach, and the bowels, causing intense pain. Never put any stronger concentration than 3% in your mouth--and be sure to spit that out--and reserve the use of 35% hydrogen peroxide for the outside of your body.
- Important notification about information and brand names used in this slideshow!
- Photo courtesy of Nathan Jones by Flickr : www.flickr.com/photos/gemsling/2688011762/
- Sean Pritchett, MD, Daniel Green, MD, and Peter Rossos, MD. Accidental ingestion of 35% hydrogen peroxide. Can J Gastroenterol. 2007 October. 20(10). 685-7.

Strychnine
A well-known poison used to kill rats, other rodents, and birds, strychnine has been used for centuries, although its chemical composition has only been known since the 1800's. Strychnine kills in minutes by paralyzing the muscles needed for breathing, its victims dying of asphyxiation. The effects of this poison are dramatic, many victims experiencing a quick sequence of severe nausea, frothing at the mouth, bulging eyes, painful muscle contractions all over their bodies, and blue skin, staying conscious to the very end. As little as 5 mg of this poison kills when it is administred by IV, as little as 30 mg when it is taken orally. Small doses have been given to athletes in Olympic competition in the mistaken belief the toxin could stimulate muscle strength; the athletes did not die, but they had to be carried out of the stadium.
- Important notification about information and brand names used in this slideshow!
- Photo courtesy of savagecats by Flickr : www.flickr.com/photos/savagecats/5421980723/
- Sharma, R.K., Concise textbook of forensic medicine & toxicology, Elsevier, 2008.

Batrachotoxins
The batrachotoxins are a group of poisonous compounds extracted from the South American dart frog. The frogs do not produce the toxins themselves. It is believed that they digest the poison from a food source, probably some kind of beetle, and then excrete it, sharing it by rubbing other frogs that may not have eaten the poisonous beetle.. Tropical frogs grown in captivity do not make the toxin. Among the most potent neurotoxins known to science, the batrachotoxins include over 100 different chemical compounds that are lethal in doses of as little as 5 micrograms, 5 millionths of a gram. There is no antidote for these toxic compounds.
- Important notification about information and brand names used in this slideshow!
- Photo courtesy of Cele4 by Wikimedia Commons : en.wikipedia.org/wiki/File:Goldenergiftfrosch1cele4.jpg
- Tokuyama T et al (1968). The structure of Batrachotoxinin A, a novel steroidal alkaloid from the Columbian arrow poison frog, Phyllobates aurotaenia. J. Am. Chem. Soc. 90 (7): 1917–1918.

VX
VX is an especially toxic nerve agent used in chemical warfare. It is known by its chemical name O-ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioate and also by its code name in the British military, Purple Possum. This chemical looks like motor oil, emitting almost no toxic fumes, making it easy to transport and hard to detect. As little as 10 mg can kill an adult in minutes, causing a rapid progression of twitching muscles at the site of contact, runny nose, contractions of the pupils of the eyes, and respiratory arrest. The antidote for VX is the nerve agent atropine, commonly available in emergency rooms, but the attending physician would have to be able to determine that VX was the poison in a very short time for treatment to be successful.
- Important notification about information and brand names used in this slideshow!
- Photo courtesy of U.S. Army Chemical Materials Agency by Wikimedia Commons : en.wikipedia.org/wiki/File:Last_VX_M55_rocket_destroyed_Tooele_Chemical_Agent_Disposal_Facility.jpg
- US Army Toxic Chemical Agent Safety Standards. (PDF). DA PAM 385-61. Section 7-8 Self/Buddy Aid Procedures. US Army.























Your thoughts on this
Loading...