The only medication for diabetes that always works, if it is taken at the right time and in the right dosage, is insulin. Other medications for diabetes stimulate the pancreas to release more insulin, or make cells more sensitive to insulin, or slow down the process of digestion so that the body does not need as much insulin as quickly as it would otherwise. Insulin itself, however, is still needed to keep sugar (and fat) moving from the bloodstream into cells.
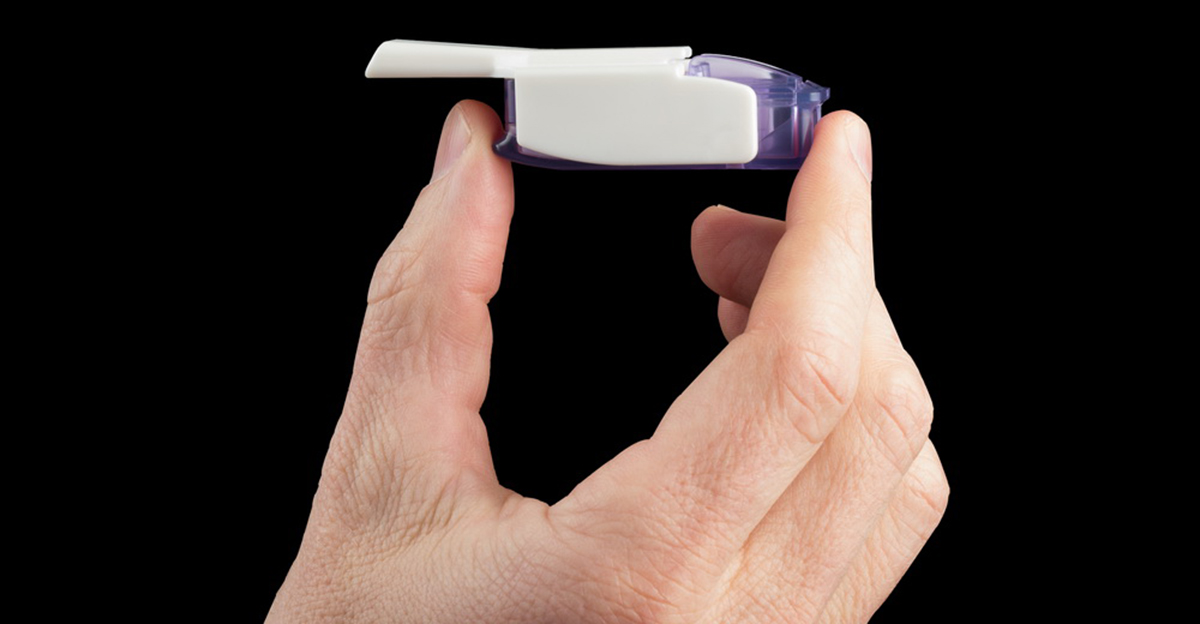
"Pills for diabetes" are not actually insulin. And aside from the fact that some diabetics just don't like getting shots, there are potential complications of insulin injections such as hitting a vein and delivering the insulin to the body too fast, or broken needles, or not filling the syringe properly and injecting a (usually harmless) air bubble. All of the issues with insulin injection may soon become moot with the introduction of a new inhaled insulin called Afrezza, developed by MannKind Corporation.
An Inventor's Passion
MannKind, based in Valencia, Calif., is run by Alfred E. Mann, a highly successful aerospace and medical device entrepreneur still very active at age 88. MiniMed, a company he started that made insulin pumps for diabetics, was sold to Medtronic for about $3 billion in 2001. Mr. Mann also started companies that developed pacemakers and cochlear implants.
Developing inhaled insulin, however, has been his passion.
“Diabetes is a major health problem in the United States,” Mann said after an FDA advisory panel gave preliminary approval to Afrezza in April 2014, “and we are committed to bring Afrezza to the many patients who might benefit from this novel product.”
Mann's commitment took the form of spending about $2.3 billion of his personal fortune on research and development of the product.
How Well Does Afrezza Work?
The studies submitted to the FDA review panel were summarized as:
"Efficacy and safety data came from studies involving a total of 3017 patients, including 1026 with type 1 and 1991 with type 2 diabetes. At 24 weeks, Afrezza reduced HbA1c levels by the prespecified end point of 0.4 percentage points in both groups. Hemoglobin A1c reduction was inferior to that of insulin aspart among type 1 patients but significantly superior to placebo among type 2 patients who were also taking oral glucose-lowering medications."
What does that mean in plain English?
See Also: Insulin: The Only Medication For Diabetes That Always Work
The benefits for using Afrezza are comparable to "adding a pill" for type 2 diabetics. It's not as good as injected Novolog for type 1 diabetics, but it's "better than nothing" for type 2 diabetics.
Should You Be Asking Your Doctor for Afrezza?
It is a little early to be asking your doctor for Afrezza. While the FDA has approved the medication for use by the general public, MannKind Corporation has not yet found a corporate partner to distribute the drug.

Large pharmaceutical firms have run into problems with inhaled insulin before.
The fact is, there were numerous problems with Exubera.
- Exubera was delivered in an inhaler about the size of a tennis ball, hard to fit in the mouth.
- The Exubera inhaler had to be pumped multiple times to charge the aerosol delivery device.
- Exubera was delivered in different units than injected insulin. Users had to learn a second system for computing dosage.
- Exubera was considerably more expensive than injected insulin, about $5 a dose compared to $2 to $3 for injected insulin.
- The needles used for injecting insulin had already become much thinner and more nearly painless than old-style insulin needles.
- Exubera was rolled out at the same time as the medication Januvia, which got similar results (even though, as pointed out before, it was not itself actually insulin).
However, assuming MannKind finds a partner to distribute the medication and together Mannkind and its partner design a comfortable inhaler that is easy to use, there are some diabetics who are especially likely to benefit from this kind of product. If you are a type 2 diabetics and you:
- Just don't like using needles,
- Aren't getting good control over your blood sugar levels just with medications such as metformin and/or Januvia,
- Aren't on any other kind of insulin, and
- Primarily have a problem controlling blood sugar levels after meals
then a product like Afrezza may be helpful. Because this brand of inhaled insulin is a fast-acting insulin like R or Novolog, it doesn't keep blood sugar levels from creeping up overnight. It may not be especially helpful when there is a problem with gastroparesis, slow digestion that causes slow release of glucose into the bloodstream. It is possible to have a hypoglycemic reaction from Afrezza if you take the product and then don't actually eat your meal, although that also happens with the injection of any other kind of fast-acting insulin.
Originally, MannKind partnered with Sanofi for the marketing and distribution of Afrezza. However, the partnership was terminated in 2016, with MannKind taking back the rights. Since then, MannKind faced challenges in effectively marketing and distributing the product on its own.
And there were reports of side effects in the clinical trials for Afrezza. Some users developed cough or sore throat. Sometimes the medication can cause throat pain.
See Also: Insulin Spray Improves Memory in Alzheimer's Patients
The FDA is ordering post-marketing tests for Afrezza to determine whether it increases the risk of lung cancer, and to study variability in how well it works for individual users and in different classes of users. The FDA has also ordered studies of the product's use by children who have diabetes.
In the meantime, some patients have reported a cough after inhaling Afrezza. This is one of the more common side effects but is generally mild and decreases over time. Some patients experience throat pain or irritation after using the product.
As with all insulins, the most common side effect of Afrezza is hypoglycemia. Symptoms of hypoglycemia can include sweating, trembling, blurred vision, rapid heartbeat, confusion, and more.
Still, if you have poorly controlled type 1 or type 2 diabetes and you just don't want to use needles, Afrezza may be the product for you. It will be only one of a number of things you need to do to get your diabetes under control, but it can be a useful part of some programs of diabetes treatment.
MannKind continue to invest in promotional activities, educational efforts, and further research to highlight the benefits of Afrezza and improve its market presence.
- Tucker ME. FDA Approves Inhaled Insulin Afrezza for Diabetes. Medscape Medical News. 14 June 2014.
- Photo courtesy of MannKind Media Room by : www.mannkindcorp.com/news-and-events-media-room-photos.htm
- Photo courtesy of MannKind Media Room by : www.mannkindcorp.com/news-and-events-media-room-photos.htm

