Table of Contents
A new treatment for psoriasis has been approved by the FDA, and it is the first of its kind that has been produced. Unlike the usual creams, lotions and tablets, Enstilar® is a foam application. For those who suffer from psoriasis, particularly the common form psoriasis vulgaris, treatment options have always been limited, and it takes a very long time to gain control of the condition, let alone clear it. To understand how the foam treatment works, you need to know a little about psoriasis.
What Is Psoriasis?
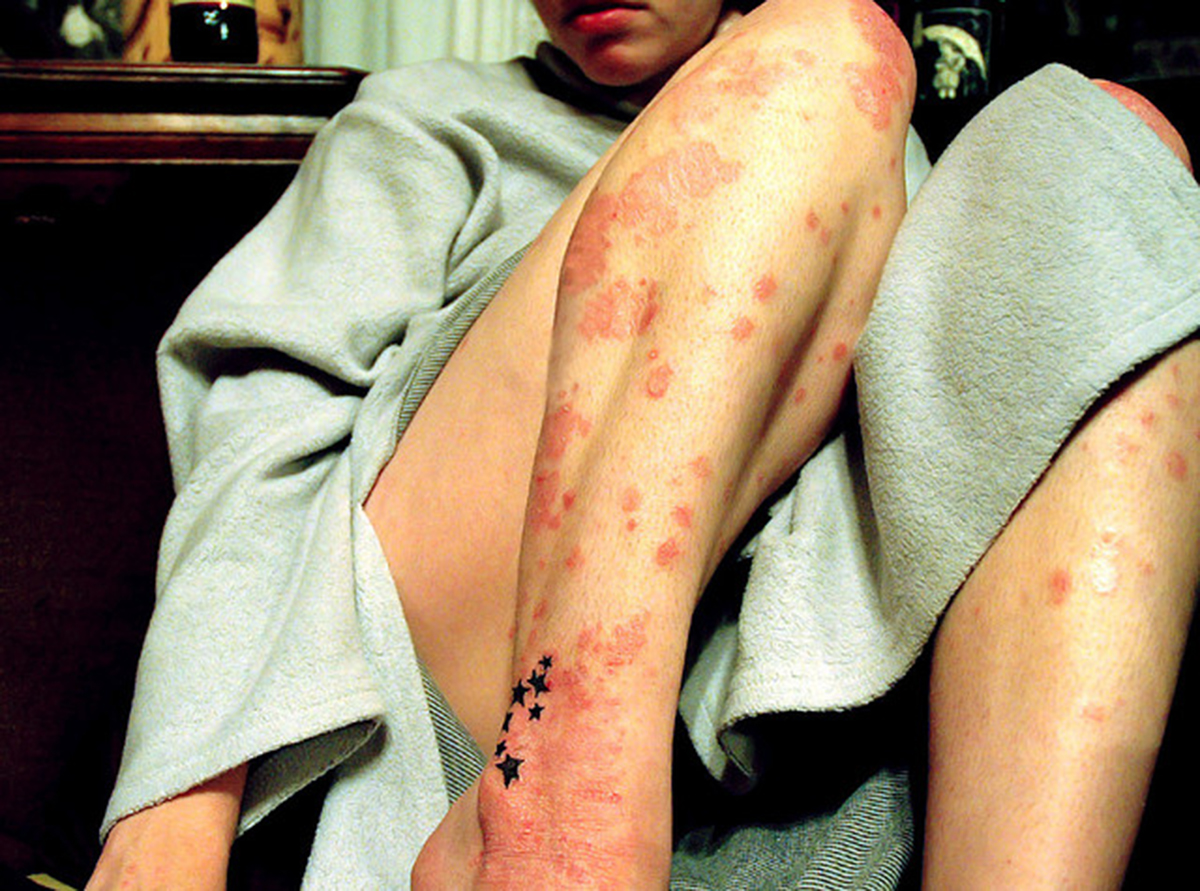
Psoriasis is an inflammatory and chronic disease of the skin. It is estimated that up to 4% of the western population have some form of psoriasis, and 8.80% of those suffer from the common psoriasis vulgaris. The disease causes the skin cells to multiply much faster than normal skin cells, and this results in reddened plagues that are raised on the skin, which are covered in white colored scales. The most common sites affected are the elbows, scalp, and knees, but it can also appear on the face and inside the ears, as well as on the torso and lower limbs. There is a form of arthritis associated with psoriasis, and up to 30% have this complication. Other complications include deformity of the finger and toenails.
What Is Enstilar®?
Medical Cautions
If you have certain pre-existing medical conditions aside from the psoriasis, it is important to discuss the option of using Enstilar® thoroughly before initiating treatment. As a precaution, the following medical issues can be a concern:
- Hypercalcemia
- Previous kidney stones
- Liver disease
- Kidney disease
- Infection of the skin
- Severe psoriasis with pus
- If you are already having phototherapy with UV light
READ Biologics - New Treatment Hope For Psoriasis Sufferers
Because Enstilar® is a topical treatment, it is unknown at this point in time whether or not it can affect a baby in the womb, or a breastfeeding baby. Your doctor may recommend you avoid contact between your skin and the baby after application, or may suggest you wait until after you have finished the pregnancy or breastfeeding before initiating treatment. There is an age restriction on using Enstilar®, and recipients of the treatment must be 12 years and over.
- Photo courtesy of mysiann: www.flickr.com/photos/mysiann/489536302/
- Photo courtesy of
- Photo courtesy of roland: www.flickr.com/photos/roland/6943626128/

