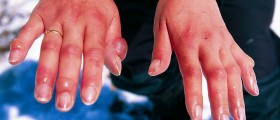
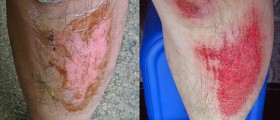
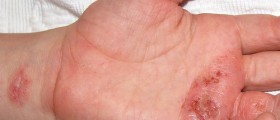
I have a MSDS for Sodium hydroxide - It does not mention vinegar. You have it backwards! It's only OK with dilute Sodium hydroxide, otherwise it causes worse burns.
Loading...
.. I have worked with Sodium Hydroxide for 14 years Vinegar is the best solution to stop the burn... Then wash in water for 15 mins.. I have had several accidents with it... Vinegar works... Most people who work with lye on a regular basis
have vinegar on the same shelf..
Loading...
hi my name is faisal. i am working in a pharmacuitical compny. raw mattrial store.i was handling a sodium hydrooxide bag.bag was closed just some of the hydrooxide touch my hands minorly...after i goes to w room to wash the hands.befor that i go to toilet.i wash the penis while using the toilet suddnly i feel something on m penis skin befor eriction was good after this i got slow erictin..i am worrying..
***this post is edited by moderator *** *** private e-mails not allowed*** Please read our Terms of Use
Loading...
This really depends on what form the caustic soda is in. If it is in solid form the procedure would be lots of water first.... then vinegar if needed. if it is in solution (such as a commercial cleaning product) then vinegar is by far the better option.
Loading...
Loading...
Actually adding water to Sodium Hydroxide can cause a very exothermic reaction, causing potentially extreme thermal burning. Best thing to use is milk, as the Sodium Hydroxide will hydrolyse the fat and protein in the milk, minimising the effect on the skin. However this can also cause an exothermic reaction and should only be used as an emergency measure. If the initial chemical burn is severe of disabling, go to the ER or call an Ambulance IMEDIATELY.
Loading...
Vinegar will neautrilise the alkalinity of the caustic soda. So vinegar will definately work
Loading...
Loading...
NO!! Read those MSDS!! Rinse with water, water and more water but don't put vinegar on it. Good grief...with water, you are trying to remove any lye from your body. You have to rinse it. Vinegar for neutralizing is recommended for surfaces...not your skin. I run a soaping group with right at 3000 members. The one thing we all use is lye. The one thing we all know is that myth of using vinegar needs to go away and people need to actually READ those MSDS. It says RINSE WITH WATER.
Loading...
Loading...
http://www.ncbi.nlm.nih.gov/m/pubmed/12711953/
This article proves that house hold vinegar helps neutralize an alkaline burn faster than flushing with water will. Since in fact house hold vinegar is a weak acid, not exceeding the hydronium concentration of 5% acetic acid.
Loading...
Loading...
When solid sodium hydroxide dissolves in water it is a highly exothermic reaction.
Loading...
Loading...